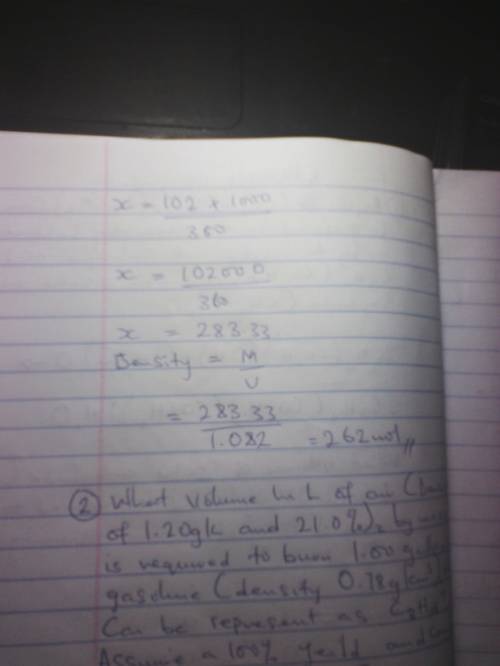Chemistry, 28.09.2019 03:30 coolkitty35
Aspirin c6h4(co2)(co2ch3),can be prepared in the chemistry laboratory by the reactions of salicylic acid, c6h4(co2h)(oh),with acetic anhydride(ch3co)2o. what volume of acetic anhydride(density,1.0820g/cm3) is required to produce 1.00kg of aspirin, assuming a 100% yield

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Aspirin c6h4(co2)(co2ch3),can be prepared in the chemistry laboratory by the reactions of salicylic...
Questions


Chemistry, 02.02.2021 19:20

Mathematics, 02.02.2021 19:20

Mathematics, 02.02.2021 19:20

Mathematics, 02.02.2021 19:20

Mathematics, 02.02.2021 19:20

Arts, 02.02.2021 19:20





History, 02.02.2021 19:20


Chemistry, 02.02.2021 19:20




Physics, 02.02.2021 19:20


Mathematics, 02.02.2021 19:20