
Chemistry, 24.07.2019 13:00 gungamer720
Consider the following reaction: 2 h2s (g) two arrows stacked on top of each other. the top arrow points to the right. the bottom arrow points to the left. 2h2 (g) + s 2 (g) which answer represents the equilibrium constant for this reaction? the concentration of h subscript two to the exponent 2 multiplied by the concentration of s2 in the numerator all over the concentration of h2s to the exponent 2 in the denominator. the concentration of h subscript two multiplied by the concentration of s2 in the numerator all over the concentration of h subscript two s in the denominator. two times the concentration of h subscript two multiplied by the concentration of s subscript two in the numerator all over two times the concentration of h subscript two s in the denominator. the concentration of hsubscript two s to the exponent 2 in the numerator all over the concentration of h subscript two multiplied by the concentration of s subscript two in the denominator.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

You know the right answer?
Consider the following reaction: 2 h2s (g) two arrows stacked on top of each other. the top arrow p...
Questions





Mathematics, 11.10.2019 01:00




Mathematics, 11.10.2019 01:00

Physics, 11.10.2019 01:00


Mathematics, 11.10.2019 01:00






History, 11.10.2019 01:00


Biology, 11.10.2019 01:00

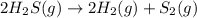
![K_c=\frac{[H_2]^2\times [S_2]}{[H_2S]^2}](/tpl/images/0127/3686/13e21.png)
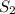 in the numerator all over the concentration of
in the numerator all over the concentration of 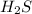 to the exponent 2 in the denominator.
to the exponent 2 in the denominator.

