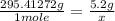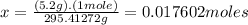Chemistry, 16.11.2019 16:31 davidsouth444
Achemist measured 5.2 g copper(ii) bromide tetrahydrate (cubr2•4( how many moles were measured out? answer in units of mole.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
Achemist measured 5.2 g copper(ii) bromide tetrahydrate (cubr2•4( how many moles were measured out?...
Questions


Biology, 03.06.2021 19:10



English, 03.06.2021 19:10

Physics, 03.06.2021 19:10

Chemistry, 03.06.2021 19:10

Mathematics, 03.06.2021 19:10

Mathematics, 03.06.2021 19:10


Mathematics, 03.06.2021 19:10

Computers and Technology, 03.06.2021 19:10




English, 03.06.2021 19:10

Chemistry, 03.06.2021 19:10

Computers and Technology, 03.06.2021 19:10


English, 03.06.2021 19:10

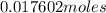
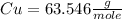
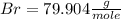
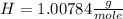
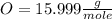
![63.546+(79.904).2+4.[2(1.00784)+15.999]=295.41272](/tpl/images/0377/5648/230da.png)