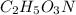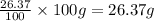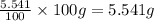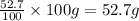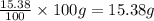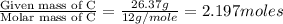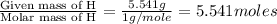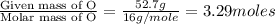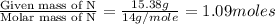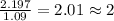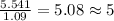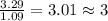Chemistry, 16.11.2019 16:31 gabegabemm1
What is the molecular formula for a compound that is 26.37 carbon 5.541 hydrogen 52.70 oxygen and 15.38 nitrogen and has a molar mass of 182.16g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

You know the right answer?
What is the molecular formula for a compound that is 26.37 carbon 5.541 hydrogen 52.70 oxygen and 15...
Questions


Social Studies, 22.09.2019 17:20


History, 22.09.2019 17:20


English, 22.09.2019 17:20


English, 22.09.2019 17:20





Mathematics, 22.09.2019 17:20

Physics, 22.09.2019 17:20

Mathematics, 22.09.2019 17:20



Biology, 22.09.2019 17:20

History, 22.09.2019 17:20

Physics, 22.09.2019 17:20