Chemistry, 25.07.2019 08:30 itcelmairani
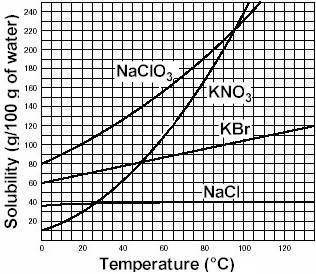
Asolution is a homogeneous mixture of one or more solutes dissolved in a solvent. a specific volume of solvent is only able to dissolve a limited amount of solute. as long as the solvent is able to dissolve more solute, the solution remains unsaturated. when the solvent can no longer dissolve additional solute, the solution is saturated. at this point, any additional solute will fall to the bottom of the container. the amount of solute that can be dissolved in a given amount of solvent at a specific temperature and pressure is defined as the solubility of the solute. consider the solubility curves of several salts in water. based on the information here, if 220 grams of the salt kbr are added to 100 ml of water at 100oc, we would label that solution as a) insoluble b) saturated c) supersaturated d) unsaturated

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
Asolution is a homogeneous mixture of one or more solutes dissolved in a solvent. a specific volume...
Questions


English, 26.06.2020 16:01




Mathematics, 26.06.2020 16:01


Biology, 26.06.2020 16:01


Mathematics, 26.06.2020 16:01




Mathematics, 26.06.2020 16:01


Mathematics, 26.06.2020 16:01