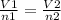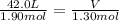Chemistry, 25.07.2019 14:00 brittany7436
Acylinder, with a piston pressing down with a constant pressure, is filled with 1.90 moles of a gas (n1), and its volume is 42.0 l (v1). if 0.600 mole of gas leak out, and the pressure and temperature remain the same, what is the final volume of the gas inside the cylinder?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3


You know the right answer?
Acylinder, with a piston pressing down with a constant pressure, is filled with 1.90 moles of a gas...
Questions

Computers and Technology, 25.02.2020 04:31


Arts, 25.02.2020 04:31


Mathematics, 25.02.2020 04:31













History, 25.02.2020 04:32