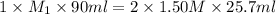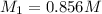Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3
You know the right answer?
Avolume of 90.0 ml of aqueous potassium hydroxide (koh) was titrated against a standard solution of...
Questions


Mathematics, 15.08.2021 02:50



English, 15.08.2021 02:50


Mathematics, 15.08.2021 02:50


Computers and Technology, 15.08.2021 02:50

History, 15.08.2021 02:50



Mathematics, 15.08.2021 02:50

Mathematics, 15.08.2021 02:50

Mathematics, 15.08.2021 02:50

Spanish, 15.08.2021 02:50

Biology, 15.08.2021 03:00

Mathematics, 15.08.2021 03:00


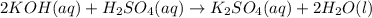
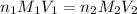
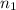 = acidity of an base = 1
= acidity of an base = 1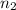 = basicity of an acid = 2
= basicity of an acid = 2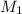 = concentration of
= concentration of 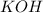 = ?
= ?
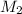 = concentration of
= concentration of 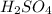 = 1.50 M
= 1.50 M
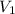 = volume of
= volume of  = volume of
= volume of