Chemistry, 26.07.2019 17:30 haileysolis5
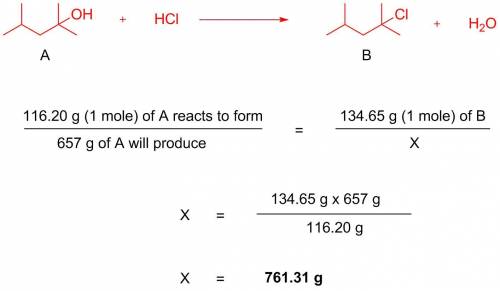
657 g of 2-chloro-2,4-dimethylpentane (g/mol = 134.65) is generated from a reaction between 2,4-dimethyl-2-pentanol (g/mol = 116.20) and excess hcl. assuming 100% yield, how many grams of 2,4-dimethyl-2-pentanol were used?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3


Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
657 g of 2-chloro-2,4-dimethylpentane (g/mol = 134.65) is generated from a reaction between 2,4-dime...
Questions




Arts, 19.10.2019 00:00


Chemistry, 19.10.2019 00:00

Mathematics, 19.10.2019 00:00

Health, 19.10.2019 00:00




English, 19.10.2019 00:00



English, 19.10.2019 00:00


Social Studies, 19.10.2019 00:00

Physics, 19.10.2019 00:00

Arts, 19.10.2019 00:00

Social Studies, 19.10.2019 00:00