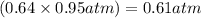Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3
You know the right answer?
Amixture of he and ne at a total pressure of 0.95 atm is found to contain 0.32 mol of he and 0.56 mo...
Questions









Biology, 30.10.2021 02:10

Chemistry, 30.10.2021 02:10

Computers and Technology, 30.10.2021 02:10


Social Studies, 30.10.2021 02:10


Mathematics, 30.10.2021 02:10

Geography, 30.10.2021 02:10


English, 30.10.2021 02:20

English, 30.10.2021 02:20

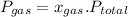
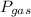 is partial pressure of a gas in mixture,
is partial pressure of a gas in mixture, 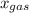 is mole fraction of a gas in mixture and
is mole fraction of a gas in mixture and 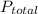 is total pressure of mixture.
is total pressure of mixture.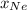 = (number of moles of Ne)/(Total number of moles in mixture)
= (number of moles of Ne)/(Total number of moles in mixture)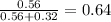
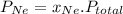 =
=