
Chemistry, 26.07.2019 20:30 wyattgrubb00
Which equivalence factor set should you use to convert from 4.23 x 1012 atoms of ca to grams of ca? a) (1 mol ca/6.02 x 1023 atoms ca)(40.08 g ca/1 mol ca) b) (1 mol ca/4.23 x 1012 atoms ca)(40.08 g ca/1 mol ca) c) (4.23 x 1012 atoms ca/1 mol ca)(1 mol ca/40.08 g ca) d) 4.23 x 1012 atoms ca/6.02 x 1023 atoms ca)(40.08 g ca)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Figure 10-1 study figure 10-1. the strong nuclear force felt by a single proton in a large nucleus
Answers: 3


Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1
You know the right answer?
Which equivalence factor set should you use to convert from 4.23 x 1012 atoms of ca to grams of ca?...
Questions

English, 26.09.2019 04:20


English, 26.09.2019 04:20


English, 26.09.2019 04:20

English, 26.09.2019 04:20




English, 26.09.2019 04:20


English, 26.09.2019 04:20

English, 26.09.2019 04:20

English, 26.09.2019 04:20


English, 26.09.2019 04:20


Chemistry, 26.09.2019 04:20


English, 26.09.2019 04:20

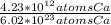 * 40.08 g Ca
* 40.08 g Ca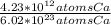 * 40.08 g Ca
* 40.08 g Ca

