
Chemistry, 28.07.2019 01:30 mallyosburn
In an exothermic reaction, the potential energy of the products is lower than that of the reactants. which of the following is also true for an exothermic reaction? the enthalpy change is negative it requires a catalyst to take place energy is absorbed from the surroundings the kinetic energy of the products is also lower

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Acycloalkane molecule contains 8 carbon atoms. how many hydrogen atoms are present in the molecule?
Answers: 2

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
In an exothermic reaction, the potential energy of the products is lower than that of the reactants....
Questions

History, 03.07.2019 13:30

Spanish, 03.07.2019 13:30


Physics, 03.07.2019 13:30

History, 03.07.2019 13:30

Mathematics, 03.07.2019 13:30



Mathematics, 03.07.2019 13:30


Chemistry, 03.07.2019 13:30

History, 03.07.2019 13:30


Biology, 03.07.2019 13:30

Mathematics, 03.07.2019 13:30





Spanish, 03.07.2019 13:30

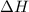 for the reaction comes out to be positive.
for the reaction comes out to be positive.


