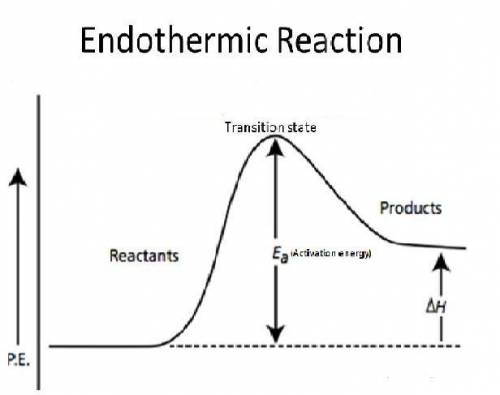
When a given reaction is conducted in a calorimeter, energy is absorbed from the surrounding water and results in a decrease in the water’s temperature. which of the following best describes the difference in the potential energy of the reactants and products in this reaction? the potential energy remains low before and after the reaction. the potential energy of the reactants is higher than that of the products. the potential energy of the reactants is much lower than that of the products the potential energy of the reactants does not differ from that of the products.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
A32 year old immigrant from a patriarchal country is giving birth. as she is delivering the baby, she tearfully confesses to her doctor that this is her 4th child and she simply cannot handle any more children. she tells the doctor that her husband refuses to use contraception or allow her to, and she begs her doctor to tie her tubes and not tell her husband. the doctor complies. was hipaa violated? why or why not?
Answers: 3


Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3
You know the right answer?
When a given reaction is conducted in a calorimeter, energy is absorbed from the surrounding water a...
Questions


Mathematics, 14.02.2021 09:10

Biology, 14.02.2021 09:10

Arts, 14.02.2021 09:10


English, 14.02.2021 09:10

Mathematics, 14.02.2021 09:10

History, 14.02.2021 09:10




Biology, 14.02.2021 09:10

History, 14.02.2021 09:10

Mathematics, 14.02.2021 09:10



Mathematics, 14.02.2021 09:10

Mathematics, 14.02.2021 09:10

Mathematics, 14.02.2021 09:10

Social Studies, 14.02.2021 09:10

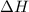 for the reaction comes out to be positive.
for the reaction comes out to be positive.