

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
The acid-dissociation constant for benzoic acid (c6h5cooh) is 6.3×10−5. calculate the equilibrium co...
Questions


Mathematics, 06.05.2020 03:37


Biology, 06.05.2020 03:37


Mathematics, 06.05.2020 03:37






Computers and Technology, 06.05.2020 03:37


Chemistry, 06.05.2020 03:37

Mathematics, 06.05.2020 03:37

Law, 06.05.2020 03:37


Mathematics, 06.05.2020 03:37


![[C_6H_5COOH]_{eq}=0.06104M](/tpl/images/0143/6976/960a5.png)
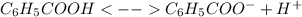
![Ka=\frac{[C_6H_5COO^-]_{eq}[H^+]_{eq}}{[C_6H_5COOH]_{eq}}](/tpl/images/0143/6976/5f347.png)
 , which alters the aforesaid equation in the following way, based on the ICE method:
, which alters the aforesaid equation in the following way, based on the ICE method: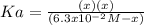
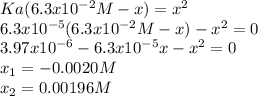
![[C_6H_5COOH]_{eq}=0.0063M-0.00196M=0.06104M](/tpl/images/0143/6976/e3473.png)


