

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1
You know the right answer?
Aluminum is produced commercially by the electrolysis of al2o3 in the presence of a molten salt. if...
Questions

Mathematics, 31.12.2019 00:31





Mathematics, 31.12.2019 00:31

Mathematics, 31.12.2019 00:31


Mathematics, 31.12.2019 00:31




Mathematics, 31.12.2019 00:31



Chemistry, 31.12.2019 00:31




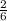 = 3.83 x 10⁴ mole of Al
= 3.83 x 10⁴ mole of Al


