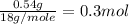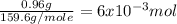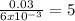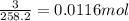Chemistry, 30.07.2019 02:00 maysahdabest
Suppose you begin with 1.50~g of the hydrate copper(ii)sulfate · x-hydrate (cuso4· x h2o), where x is an integer. after dehydration you find that you are left with 0.96~g of the an-hydrate cuso4. what is the unknown integer x. round your answer to the nearest integer, enter only an integer. suppose you begin with of the hydrate kal(so4)2 · 12h2o. after dehydration you find that you are left with 3.0~g of the an-hydrate kal(so4)2. how many grams did you start with? write the value to the correct number of significance figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1


Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 04:20
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1
You know the right answer?
Suppose you begin with 1.50~g of the hydrate copper(ii)sulfate · x-hydrate (cuso4· x h2o), where x i...
Questions



Mathematics, 23.07.2019 05:30


Mathematics, 23.07.2019 05:30


Mathematics, 23.07.2019 05:30


Advanced Placement (AP), 23.07.2019 05:30

Mathematics, 23.07.2019 05:30



Advanced Placement (AP), 23.07.2019 05:30


Chemistry, 23.07.2019 05:30

Social Studies, 23.07.2019 05:30

Mathematics, 23.07.2019 05:30

History, 23.07.2019 05:30