
Chemistry, 30.07.2019 02:00 razielcornils04
This reaction was monitored as a function of time: ab --> a + b a plot of 1/[ab] versus time yields a straight line with slope of -0.055/m*s. a) what is the value of the rate constant (k) for this reaction t this temperature? b) write the rate law for the reaction. c) what is the half-life when the initial concentration is 0.55m? d) if the initial concentration of ab is 0.250m, and the reaction mixture initially contains no products, what are the concentrations of a and b after 75s?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which statement about sound is not true? a. air particles travel with sound waves. b. sound waves cannot travel through a vacuum. c. sound waves exist even if no one hears them. d. air particles vibrate along the path of a sound wave.
Answers: 1

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
This reaction was monitored as a function of time: ab --> a + b a plot of 1/[ab] versus time yi...
Questions


Mathematics, 03.02.2021 16:20

Mathematics, 03.02.2021 16:20



Mathematics, 03.02.2021 16:20

Mathematics, 03.02.2021 16:20



Mathematics, 03.02.2021 16:20

Geography, 03.02.2021 16:20

Business, 03.02.2021 16:20

History, 03.02.2021 16:20

Mathematics, 03.02.2021 16:20



Biology, 03.02.2021 16:20

Mathematics, 03.02.2021 16:20


Biology, 03.02.2021 16:20

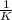 .
. 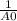
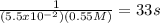
![\frac{1}{t} [ \frac{1}{[A]} - \frac{1}{[Ao]} ]](/tpl/images/0148/8063/927e3.png)


