
Chemistry, 03.08.2019 15:30 dezmondpowell
Asolution is prepared by dissolving 20.2 ml of methanol (ch3oh) in 100.0 ml of water at 25oc. the final volume of the solution is 118 ml. the densities of methanol and water at this temperature are 0.782 g/ml and 1.00 g/ml, respectively. for this solution, calculate the molarity, molality, percent by mass, mole fraction, and mole percent.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
Asolution is prepared by dissolving 20.2 ml of methanol (ch3oh) in 100.0 ml of water at 25oc. the fi...
Questions





Mathematics, 17.09.2019 22:30




English, 17.09.2019 22:30

Mathematics, 17.09.2019 22:30

Mathematics, 17.09.2019 22:30

Mathematics, 17.09.2019 22:30



Mathematics, 17.09.2019 22:30

Mathematics, 17.09.2019 22:30


Physics, 17.09.2019 22:30

Mathematics, 17.09.2019 22:30

Mathematics, 17.09.2019 22:30

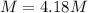
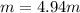
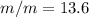 %
%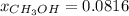
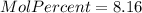 %
%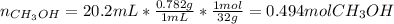
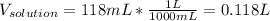
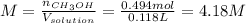
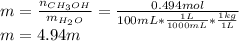
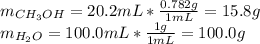
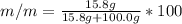 %
%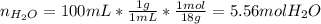
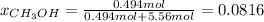
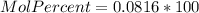 %
%

