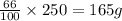Chemistry, 03.08.2019 15:30 franky2871
An aqueous solution of licl is 34.0 % licl. what mass of water is present in 250.0 g of the solution? (density of water is 1.00 g/ml) (solution contains only licl and water.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
An aqueous solution of licl is 34.0 % licl. what mass of water is present in 250.0 g of the solution...
Questions

Mathematics, 24.06.2019 15:30

History, 24.06.2019 15:30

Biology, 24.06.2019 15:30

Physics, 24.06.2019 15:30

Biology, 24.06.2019 15:30

Social Studies, 24.06.2019 15:30





Biology, 24.06.2019 15:30




English, 24.06.2019 15:30

Mathematics, 24.06.2019 15:30

Physics, 24.06.2019 15:30



Biology, 24.06.2019 15:30