
Chemistry, 01.08.2019 01:30 little68941
At a particular temperature, 12.0 moles of so3 is placed into a 3.0-l rigid container, and the so3 dissociates by the reaction 2so3(g) 34 2so2(g) 1 o2(g) at equilibrium, 3.0 moles of so2 is present. calculate k for this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
At a particular temperature, 12.0 moles of so3 is placed into a 3.0-l rigid container, and the so3 d...
Questions



History, 28.06.2019 02:30

Mathematics, 28.06.2019 02:30


Computers and Technology, 28.06.2019 02:30




Biology, 28.06.2019 02:30


Spanish, 28.06.2019 02:30




English, 28.06.2019 02:30

Health, 28.06.2019 02:30


Mathematics, 28.06.2019 02:30

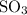 dissociate to form
dissociate to form 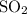 and
and 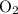 .
. 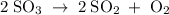
![\rm \dfrac{[SO_2]^2\;[O_2]}{[SO_3]^2}](/tpl/images/0156/4205/3de93.png)
![\rm \dfrac{[3\;moles/\;3\;L]^2\;[1.5\;moles\;/\;3\;L]}{[9\;moles\;/3\;L]^2}](/tpl/images/0156/4205/76bb9.png)



