
Chemistry, 28.06.2019 02:30 jobucks9976
Fe3+(aq) (yellow) + scn-(aq) (colorless) fescn2+(aq) (blood-red) chloride ions are colorless. potassium ions are also colorless. the above equilibrium can be created by mixing an iron (iii) chloride solution with a potassium thiocyanate solution. based on this information and the colors in the equilibrium above answer the first two questions. 1. what color would an fecl3 solution be? 2. what color would a kscn solution be? 3. what color do you get when you mix fecl3 and kscn as shown in the video for the control test tube?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2
You know the right answer?
Fe3+(aq) (yellow) + scn-(aq) (colorless) fescn2+(aq) (blood-red) chloride ions are colorless. potass...
Questions

History, 06.10.2019 21:30

Social Studies, 06.10.2019 21:30


Chemistry, 06.10.2019 21:30

Biology, 06.10.2019 21:30


Mathematics, 06.10.2019 21:30

Computers and Technology, 06.10.2019 21:30

Biology, 06.10.2019 21:30


Mathematics, 06.10.2019 21:30

History, 06.10.2019 21:30

Mathematics, 06.10.2019 21:30

Mathematics, 06.10.2019 21:30





Chemistry, 06.10.2019 21:30

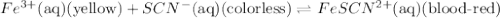
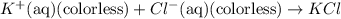
![FeCl_3+K(SCN)\rightleftharpoons KCl+[Fe(SCN)]Cl_2(blood-red)](/tpl/images/0025/5430/e3b23.png)
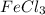 we will get blood red color solution of
we will get blood red color solution of ![[FeSCN]Cl_2](/tpl/images/0025/5430/ecffe.png) .
.

