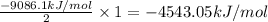Chemistry, 29.07.2019 12:50 loistaylor1819
Pentaborane b5h9(s) burns vigorously in o2 to give b2o3(s) and h2o(l). what is δh° for the combustion of 1 mol of b5h9(s)? substance δh°f (kj/mol) b2o3(s) –1273.5 b5h9(s) +73.2 h2o(l) –285.8

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Pentaborane b5h9(s) burns vigorously in o2 to give b2o3(s) and h2o(l). what is δh° for the combustio...
Questions



Mathematics, 03.11.2020 17:40

Mathematics, 03.11.2020 17:40



English, 03.11.2020 17:40

Mathematics, 03.11.2020 17:40









Mathematics, 03.11.2020 17:40



Mathematics, 03.11.2020 17:40

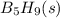 is -4543.05 kJ/mol
is -4543.05 kJ/mol
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0146/7209/45485.png)
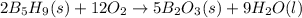
![\Delta H^o_{rxn}=[(5\times \Delta H^o_f_{(B_2O_3)})+(9\times \Delta H^o_f_{(H_2O)})]-[(2\times \Delta H^o_f_{(B_5H_9)})+(12\times \Delta H^o_f_{(O_2)})]](/tpl/images/0146/7209/08949.png)
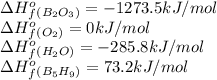
![\Delta H^o_{rxn}=[(5\times (-1273.5))+(9\times (-285.8))]-[(2\times (73.2))+(12\times 0)]\\\\\Delta H^o_{rxn}=-9086.1kJ/mol](/tpl/images/0146/7209/f092e.png)
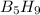 will be =
will be =