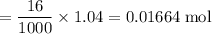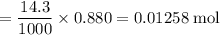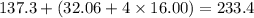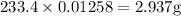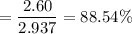Chemistry, 20.07.2019 11:10 isabelcasillas
A16.0 ml sample of a 1.04 m potassium sulfate solution is mixed with 14.3 ml of a 0.880 m barium nitrate solution and this precipitation reaction occurs: k 2 s o 4 (aq)+ba(n o 3 ) 2 (aq)→bas o 4 (s)+2kn o 3 (aq) the solid bas o 4 is collected, dried, and found to have a mass of 2.60 g . determine the limiting reactant, the theoretical yield, and the percent yield.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1


Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
A16.0 ml sample of a 1.04 m potassium sulfate solution is mixed with 14.3 ml of a 0.880 m barium nit...
Questions

Mathematics, 06.01.2021 02:20



Mathematics, 06.01.2021 02:20


English, 06.01.2021 02:20


English, 06.01.2021 02:20




English, 06.01.2021 02:20

Mathematics, 06.01.2021 02:20

Mathematics, 06.01.2021 02:20


Mathematics, 06.01.2021 02:20

Computers and Technology, 06.01.2021 02:20

Advanced Placement (AP), 06.01.2021 02:20

Mathematics, 06.01.2021 02:20