
PLZ HELP Stoichiometry Calculation
Imagine that you are solving the following stoichiometry problem:
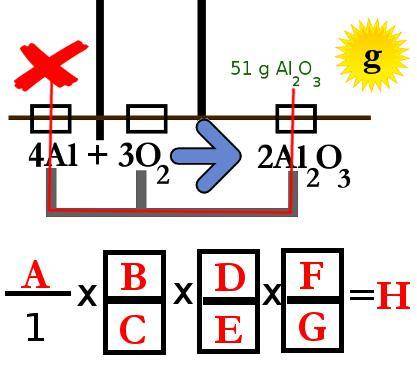
When aluminum oxidizes in air, it forms aluminum oxide (Al2O3):
4Al + 3O2 --> 2Al2O3 if a 51g sheet of aluminum oxide formed completely in excess oxygen, how many grams of aluminum were oxidized? Using the magic mole method, you diagram the problem, map out your solution route, and convert your diagram to an equation.
Use this diagram to answer the problems below.
(diagram included
2.What units go in spot C in the equation above?
mol Al2O3. mol. g. g Al2O3
3.What units go in spot B in the equation above?
g Al. mol Al2O3. mol Al. g Al2O3
4.What number goes in spot D in the equation? Enter the number only without any units
5.What number goes in spot E in the equation above? (Enter the number only

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following best defines homeostasis? forming identical cells breaking down glucose maintaining stable internal conditions increasing an organism's temperature
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
PLZ HELP Stoichiometry Calculation
Imagine that you are solving the following stoichiometry proble...
Questions

Mathematics, 10.03.2020 22:35





Computers and Technology, 10.03.2020 22:35

Mathematics, 10.03.2020 22:35



Health, 10.03.2020 22:35




Health, 10.03.2020 22:35



Biology, 10.03.2020 22:35

Engineering, 10.03.2020 22:35




