
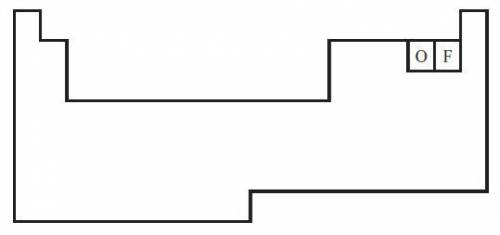
The diagram below shows a partial periodic table.
The electron configuration of oxygen is 1s2 2s2 2p4. On the periodic table, fluorine is one space to the right of oxygen. Which of the following electron configurations represents fluorine?
1s2 2s2 2p3
1s2 2s2 2p6 1s2 3s2 3p3
1s2 2s2 2p5

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 07:00
Agas has an empirical formula ch4. 0.16g of the gas occupies a volume of 240cm^3 what is the molecular formula of the me anyone who !
Answers: 1

Chemistry, 23.06.2019 11:00
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2

Chemistry, 23.06.2019 16:30
In chile, the deepest earthquake occurred at 61.7°w longitude at a depth of 540 km. if the rocks at the focus began subducting 10 million years ago and are now 1000 km from their original position, what is the average rate of subduction in cm/yr?
Answers: 1
You know the right answer?
The diagram below shows a partial periodic table.
The electron configuration of oxygen is 1s2 2s2...
Questions



Biology, 19.09.2019 17:30



Computers and Technology, 19.09.2019 17:30






English, 19.09.2019 17:30

English, 19.09.2019 17:30





Mathematics, 19.09.2019 17:30




