
Chemistry, 17.10.2021 08:10 nicolecoulthard
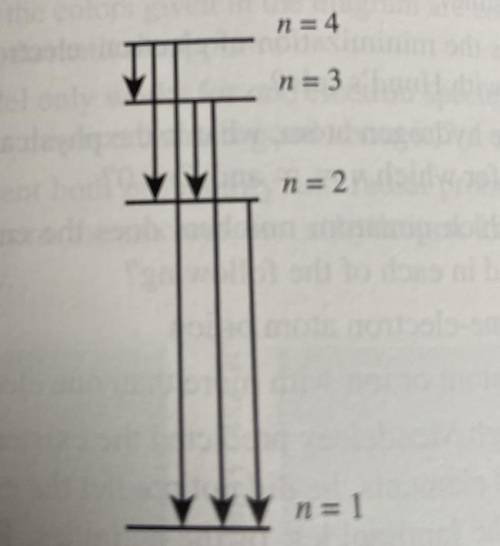
Consider only the transitions involving the first four energy levels for a hydrogen atom:
a) How many emissions are possible for an electron in the n=4 level as it goes to the ground state
b)which electronic transition is the lowest energy?
c) Which electronic transition corresponds to the shortest wavelength emission

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
Consider only the transitions involving the first four energy levels for a hydrogen atom:
a) How m...
Questions


Computers and Technology, 10.03.2020 08:53

Mathematics, 10.03.2020 08:53






Mathematics, 10.03.2020 08:54



Computers and Technology, 10.03.2020 08:54


Computers and Technology, 10.03.2020 08:54






Mathematics, 10.03.2020 08:55



