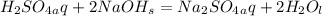Problem PageQuestion Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . Suppose 91. g of sulfuric acid is mixed with 116. g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 23.06.2019 15:00
20 look at the clock and the data table below. based on the data and on your knowledge of potential and kinetic energy, what is the best conclusion you can make about potential and kinetic energy? the total amount of energy stays the same. the clock has the most potential energy at point b since it is moving the fastest. there is always more potential energy than kinetic energy. potential energy can never be 0, but you can have 0 kinetic energy.
Answers: 1
You know the right answer?
Problem PageQuestion Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous...
Questions

Mathematics, 13.12.2021 04:10






Mathematics, 13.12.2021 04:10


Mathematics, 13.12.2021 04:10

Mathematics, 13.12.2021 04:10






Mathematics, 13.12.2021 04:10

Physics, 13.12.2021 04:10

Mathematics, 13.12.2021 04:10


Mathematics, 13.12.2021 04:10