
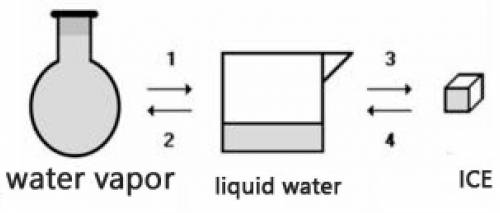
Consider the transformations a water sample undergoes without external pressure variation
(a) Transformations 2 and 4 are endothermic.
(b) Transformations 1 and 2 are exothermic.
(c) The amount of energy absorbed in 3 is equal to the amount released in 1.
(d) The amount of energy released in 2 is equal to the amount released in 4.
(e) The change of physical state does not involve heat energy.

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
What is the molarity of a solution that is prepared by adding 57.1 g of toluene (c 7 h 8 ) (density = 0.867 g/ml) to a 250 ml volumetric flask, and then filling to the mark with benzene (c 6 h 6 ) (density = 0.876 g/ml)?
Answers: 1

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
You know the right answer?
Consider the transformations a water sample undergoes without external pressure variation
(a) Trans...
Questions

Mathematics, 15.04.2021 21:30

Mathematics, 15.04.2021 21:30

Chemistry, 15.04.2021 21:30


Mathematics, 15.04.2021 21:30

Mathematics, 15.04.2021 21:30


English, 15.04.2021 21:30


World Languages, 15.04.2021 21:30


History, 15.04.2021 21:30


Mathematics, 15.04.2021 21:30

Mathematics, 15.04.2021 21:30

Mathematics, 15.04.2021 21:30

Mathematics, 15.04.2021 21:30

History, 15.04.2021 21:30




