
Chemistry, 15.07.2021 14:50 triciajfive
Consider the transformations a water sample undergoes without external pressure variation
(a) Transformations 2 and 4 are endothermic.
(b) Transformations 1 and 2 are exothermic.
(c) The amount of energy absorbed in 3 is equal to the amount released in 1.
(d) The amount of energy released in 2 is equal to the amount released in 4.
(e) The change of physical state does not involve heat energy.
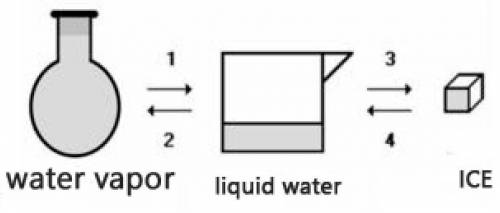

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

You know the right answer?
Consider the transformations a water sample undergoes without external pressure variation
(a) Trans...
Questions

Biology, 06.09.2020 03:01


Mathematics, 06.09.2020 03:01


Mathematics, 06.09.2020 03:01

Advanced Placement (AP), 06.09.2020 03:01

Mathematics, 06.09.2020 03:01

History, 06.09.2020 03:01

English, 06.09.2020 03:01


Chemistry, 06.09.2020 03:01



Mathematics, 06.09.2020 03:01

Chemistry, 06.09.2020 03:01

Geography, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01



Chemistry, 06.09.2020 03:01



