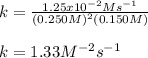NO2 (g) reacts with O2 4 NO2 (g) + O2(g) to produce N2 O5 (g) as shown by the equation below: (g) = 2 N2 O5 (g).
Experimentally the rate orders were determined and rate law written as shown below: Rate = k [NO2]2[O2 ].
Calculate the value of k if the initial concentration of NO2 was 0.250 M and initial
concentration of O2 (g) was 0.150 M. The initial rate was 1.25 x 10-2 M. s-1 .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
NO2 (g) reacts with O2 4 NO2 (g) + O2(g) to produce N2 O5 (g) as shown by the equation below: (g) =...
Questions

Mathematics, 04.08.2019 07:00

History, 04.08.2019 07:00



Biology, 04.08.2019 07:00


Health, 04.08.2019 07:00





History, 04.08.2019 07:00


Social Studies, 04.08.2019 07:00



English, 04.08.2019 07:00


Biology, 04.08.2019 07:00

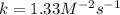
![k=\frac{r}{[NO_2]^2[O_2]}](/tpl/images/1392/3789/849e7.png)