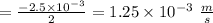Chemistry, 11.07.2021 18:50 sammuelanderson1371
Consider the reaction “2 SO2 (g) + O2 (g) = 2 SO3 was 0.175 M. After 50 s the concentration of SO2 Date: (g)”. Initial concentration of SO2 (g) (g) became 0.0500 M. Calculate rate of the reaction

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
Consider the reaction “2 SO2 (g) + O2 (g) = 2 SO3 was 0.175 M. After 50 s the concentration of SO2 D...
Questions


History, 16.01.2021 01:00


Mathematics, 16.01.2021 01:00



Mathematics, 16.01.2021 01:00

Biology, 16.01.2021 01:00


Biology, 16.01.2021 01:00



Mathematics, 16.01.2021 01:00


Mathematics, 16.01.2021 01:00



Mathematics, 16.01.2021 01:00


Mathematics, 16.01.2021 01:00

 "
"![=-\frac{1}{2} \frac{\Delta [SO_2]}{\Delta t} =-\frac{\Delta [O_2]}{\Delta t}= +\frac{1}{2} \frac{\Delta [SO_3]}{\Delta t}\\\\=\frac{\Delta [SO_2]}{\Delta t}=\frac{0.0500-0.175\ M}{505}= -2.5 \times 10^{-3} \ \frac{m}{s}\\\\](/tpl/images/1392/3786/a8505.png)