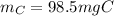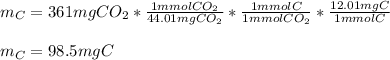Chemistry, 10.07.2021 15:00 cassidy32504
a pure sample of a compound on combustion analysis gave 361 mg of CO2 and 147mg of H2O. if the weight of the sample is 202 mg calculate the weight of carbon in the sample

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
a pure sample of a compound on combustion analysis gave 361 mg of CO2 and 147mg of H2O. if the weigh...
Questions


Mathematics, 29.10.2019 22:31

Mathematics, 29.10.2019 22:31


Physics, 29.10.2019 22:31

Mathematics, 29.10.2019 22:31


Mathematics, 29.10.2019 22:31



Mathematics, 29.10.2019 22:31


Social Studies, 29.10.2019 22:31

Computers and Technology, 29.10.2019 22:31


Biology, 29.10.2019 22:31

Mathematics, 29.10.2019 22:31

Health, 29.10.2019 22:31

English, 29.10.2019 22:31

Biology, 29.10.2019 22:31