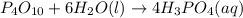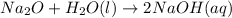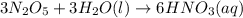Chemistry, 08.07.2021 16:00 myhomeacc32
Predict the products of each reaction, and whether the solution at equilibrium will be acidic, basic, or neutral.1. P4O10 + 6H2O (l)>2. Na2O + H2O(l) >3. N2O5 + 3H2O (l)>

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Predict the products of each reaction, and whether the solution at equilibrium will be acidic, basic...
Questions

Mathematics, 08.03.2021 14:10

Mathematics, 08.03.2021 14:10


Mathematics, 08.03.2021 14:10

Mathematics, 08.03.2021 14:10


Mathematics, 08.03.2021 14:10


Mathematics, 08.03.2021 14:10



Mathematics, 08.03.2021 14:10

Computers and Technology, 08.03.2021 14:10

Social Studies, 08.03.2021 14:10

History, 08.03.2021 14:10

Geography, 08.03.2021 14:10




Mathematics, 08.03.2021 14:10