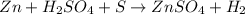Chemistry, 24.06.2021 15:50 chancecharles9oug353
In the following reaction, Zn is Zn(s) H2SO4(aq) --> ZnSO4(aq) H2(g) A. Reduced B. Oxidized C. This is not a redox reaction D. An oxidizing agent

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
In the following reaction, Zn is Zn(s) H2SO4(aq) --> ZnSO4(aq) H2(g) A. Reduced B. Oxidized C. Th...
Questions


Mathematics, 11.05.2021 21:00


Physics, 11.05.2021 21:00

History, 11.05.2021 21:00

Mathematics, 11.05.2021 21:00

Mathematics, 11.05.2021 21:00





History, 11.05.2021 21:00

Health, 11.05.2021 21:00


Mathematics, 11.05.2021 21:00


Mathematics, 11.05.2021 21:00



Mathematics, 11.05.2021 21:00