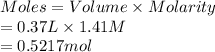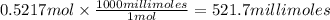Chemistry, 24.06.2021 15:50 brooklynpage5283
A chemist adds 370.0mL of a 1.41/molL potassium iodide KI solution to a reaction flask. Calculate the millimoles of potassium iodide the chemist has added to the flask. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 23.06.2019 12:00
Asubstance that will change shape to fit its container but has a definite volume is in a phase of matter
Answers: 1
You know the right answer?
A chemist adds 370.0mL of a 1.41/molL potassium iodide KI solution to a reaction flask. Calculate th...
Questions


Health, 23.08.2021 23:10




English, 23.08.2021 23:10


Mathematics, 23.08.2021 23:10


English, 23.08.2021 23:10







Computers and Technology, 23.08.2021 23:10