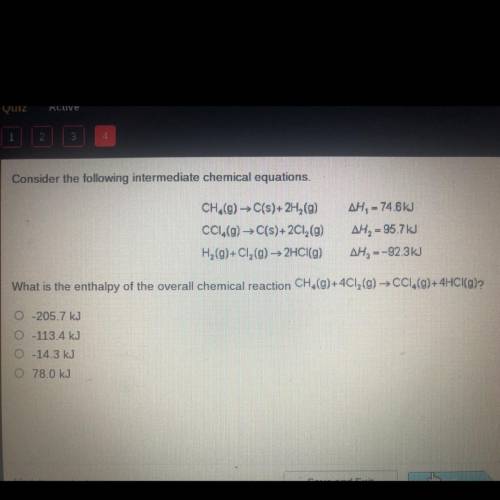
Consider the following intermediate chemical equations.
CH.(g) → C(s)+2H (9)
CC1.(g) → C(s)+2...

Chemistry, 23.06.2021 19:50 nataliajaquez02
Consider the following intermediate chemical equations.
CH.(g) → C(s)+2H (9)
CC1.(g) → C(s)+2Cl2(g)
H2(g)+C1, (g) → 2HCl(g)
AH, = 74.6 kJ
AH, = 95.7 kJ
AH, =-92.3kJ
What is the enthalpy of the overall chemical reaction CH,(g)+4C12(g) → CC1,(9)+4HCI(g)?
O-205.7 kJ
0-113.4 kJ
-14.3 kJ
0 78.0 kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Questions

Mathematics, 26.06.2019 09:30


Mathematics, 26.06.2019 09:30

History, 26.06.2019 09:30

Social Studies, 26.06.2019 09:30



History, 26.06.2019 09:30



English, 26.06.2019 09:30


Mathematics, 26.06.2019 09:30


Mathematics, 26.06.2019 09:30

Social Studies, 26.06.2019 09:30

Mathematics, 26.06.2019 09:30

Mathematics, 26.06.2019 09:30


Mathematics, 26.06.2019 09:30



