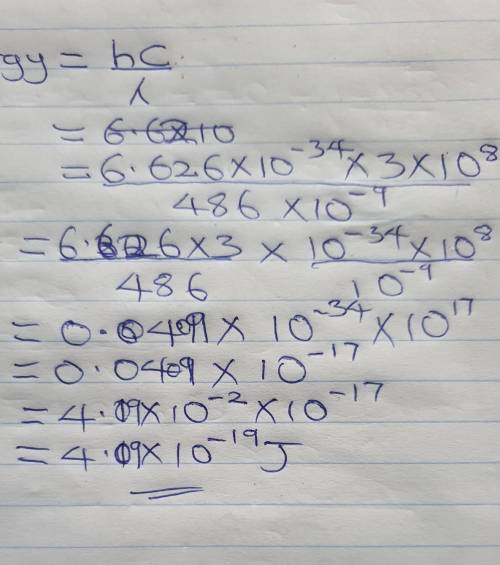The hydrogen emission spectrum is shown below. What is the energy of the
486 nm emission line? (The speed of light in a vacuum is 3.00 x 10^8 m/s, and Planck's constant is 6.626 x 10^-34 Jos.)
A. 2.44 x 10^18 J
B. 7.33 x 10^26 J
C. 6.17 x 10^14 J
D. 4.09 x 10^-19 J

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
The hydrogen emission spectrum is shown below. What is the energy of the
486 nm emission line? (The...
Questions

Mathematics, 01.10.2019 16:00










Arts, 01.10.2019 16:00

Mathematics, 01.10.2019 16:00

Mathematics, 01.10.2019 16:00

Mathematics, 01.10.2019 16:00


History, 01.10.2019 16:00

Physics, 01.10.2019 16:00



English, 01.10.2019 16:00