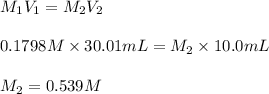g Suppose you are titrating vinegar, which is an acetic acid solution of unknown concentration, with a sodium hydroxide solution according to the equation H C 2 H 3 O 2 + N a O H ⟶ H 2 O + N a C 2 H 3 O 2 If you require 30.01 mL of 0.1798 M N a O H solution to titrate 10.0 mL of H C 2 H 3 O 2 solution, what is the molar concentration of acetic acid in the vinegar? Type

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1


Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 11:30
Which part of the healthcare system could best explain how a pharmaceutical drug works
Answers: 2
You know the right answer?
g Suppose you are titrating vinegar, which is an acetic acid solution of unknown concentration, with...
Questions



English, 15.06.2021 18:00


Mathematics, 15.06.2021 18:00


English, 15.06.2021 18:00

English, 15.06.2021 18:00


Chemistry, 15.06.2021 18:00




Mathematics, 15.06.2021 18:00

Mathematics, 15.06.2021 18:00

English, 15.06.2021 18:00




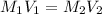
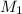 and
and 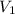 are the concentration and volume of base.
are the concentration and volume of base.
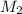 and
and 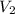 are the concentration and volume of an acid.
are the concentration and volume of an acid.