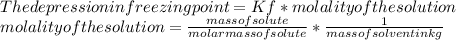Chemistry, 23.06.2021 02:50 jeffmacdonald1976
Suppose that you add 29.2 g of an unknown molecular compound to 0.250 kg of benzene, which has a K f of 5.12 oC/m. With the added solute, you find that there is a freezing point depression of 2.78 oC compared to pure benzene. What is the molar mass (in g/mol) of the unknown compound

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2



You know the right answer?
Suppose that you add 29.2 g of an unknown molecular compound to 0.250 kg of benzene, which has a K f...
Questions

English, 28.08.2021 05:10



Mathematics, 28.08.2021 05:10

English, 28.08.2021 05:10


Mathematics, 28.08.2021 05:10



Mathematics, 28.08.2021 05:10

Engineering, 28.08.2021 05:10

History, 28.08.2021 05:10

Physics, 28.08.2021 05:10


Mathematics, 28.08.2021 05:10

Health, 28.08.2021 05:10


Mathematics, 28.08.2021 05:10

History, 28.08.2021 05:10