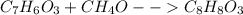Chemistry, 12.06.2021 02:10 kekoanabor19
Methyl salicylate (oil of wintergreen) is prepared by heating salicylic acid, , with methanol, . In an experiment, 1.60 g of salicylic acid is reacted with 15.0 g of methanol. The yield of methyl salicylate, , is 1.66 g. What is the percentage yield

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
Methyl salicylate (oil of wintergreen) is prepared by heating salicylic acid, , with methanol, . In...
Questions

Mathematics, 27.07.2021 01:00


Mathematics, 27.07.2021 01:00



Computers and Technology, 27.07.2021 01:00


Computers and Technology, 27.07.2021 01:00




History, 27.07.2021 01:10

Mathematics, 27.07.2021 01:10




Mathematics, 27.07.2021 01:10