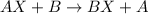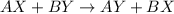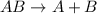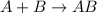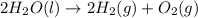Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
The electrolysis of water has the equation 2H2O (l) → 2H2 (g) + O2 (g). What type of reaction is thi...
Questions



Mathematics, 07.04.2021 23:20

Mathematics, 07.04.2021 23:20

Mathematics, 07.04.2021 23:20


Mathematics, 07.04.2021 23:20

Mathematics, 07.04.2021 23:20

Mathematics, 07.04.2021 23:20

Spanish, 07.04.2021 23:20

Mathematics, 07.04.2021 23:20

English, 07.04.2021 23:20

Mathematics, 07.04.2021 23:20

Physics, 07.04.2021 23:20


Mathematics, 07.04.2021 23:20

English, 07.04.2021 23:20

Mathematics, 07.04.2021 23:20


Mathematics, 07.04.2021 23:20