Chemistry, 11.06.2021 22:50 tyneshiajones124
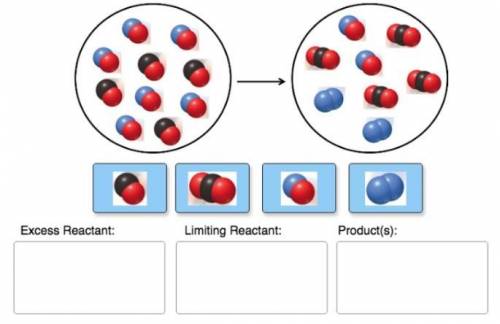
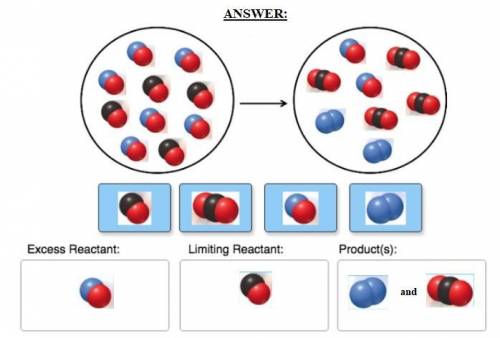
Consider the reaction of NO and CO to form N2 and CO2, according to the balanced equation: 2 NO (g) + 2 CO (g) → N2 (g) + 2 CO2 (g) Identify the excess reactant, the limiting reactant, and the product(s) using the molecular art. (Black spheres are carbon, blue spheres are nitrogen, and red spheres are oxygen.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Consider the reaction of NO and CO to form N2 and CO2, according to the balanced equation: 2 NO (g)...
Questions


Spanish, 23.04.2021 01:00



Chemistry, 23.04.2021 01:00

Biology, 23.04.2021 01:00




Mathematics, 23.04.2021 01:00



English, 23.04.2021 01:00

Mathematics, 23.04.2021 01:00

Mathematics, 23.04.2021 01:00

Mathematics, 23.04.2021 01:00

Mathematics, 23.04.2021 01:00


Mathematics, 23.04.2021 01:00

Social Studies, 23.04.2021 01:00

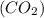 compound
compound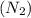
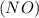 compound
compound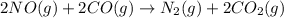
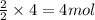 of NO
of NO