Chemistry, 11.06.2021 06:50 smcardenas02
What is the delta H when 72.0 grams H2O condenses at 100.00C?
Here are some constants that you MAY need.
specific heats heat of fusion heat of vaporization
H2O(s) = 2.1 J/g0C 6.01 kJ/mole 40.7 kJ/mole
H2O(L) = 4.18 J/g0C
H2O(g) = 1.7 J/g0C
2930 kJ
163 kJ
-163 kJ
-2930 kJ

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
When molecules of water vapor in the air come in contact with a cold can of soft drink , they lose energy ,slow down,and form a liquid due to decrease in ?
Answers: 2

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3
You know the right answer?
What is the delta H when 72.0 grams H2O condenses at 100.00C?
Here are some constants that you MAY...
Questions


Health, 18.03.2021 03:20









Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20


History, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

English, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

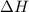 is -163 kJZ
is -163 kJZ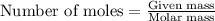 ......(1)
......(1)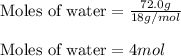
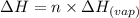 ......(2)
......(2)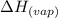 = specific heat of vaporization = -40.7 kJ/mol
= specific heat of vaporization = -40.7 kJ/mol