
Chemistry, 10.06.2021 21:30 xxgissellexx
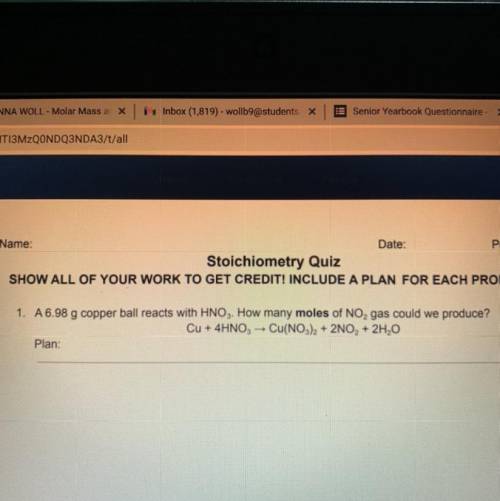
A 6.98 g copper ball reacts with HNO3. How many moles of NO, gas could we produce? Cu + 4HNO3 → Cu(NO3)2 + 2NO, + 2H2O

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3
You know the right answer?
A 6.98 g copper ball reacts with HNO3. How many moles of NO, gas could we produce?
Cu + 4HNO3 → Cu(...
Questions

Mathematics, 16.02.2021 01:00

Advanced Placement (AP), 16.02.2021 01:00


Mathematics, 16.02.2021 01:00

History, 16.02.2021 01:00





Spanish, 16.02.2021 01:00

Mathematics, 16.02.2021 01:00



English, 16.02.2021 01:00

Social Studies, 16.02.2021 01:00


Health, 16.02.2021 01:00






