Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Note the ph and poh values labeled with letters on the ph scale below. based on log rules and the way ph is calculated, what is the difference in [oh– ] concentration between point a and point b. a) 10^1 b) 10^5 c) 10^6 d) 10^7
Answers: 1

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
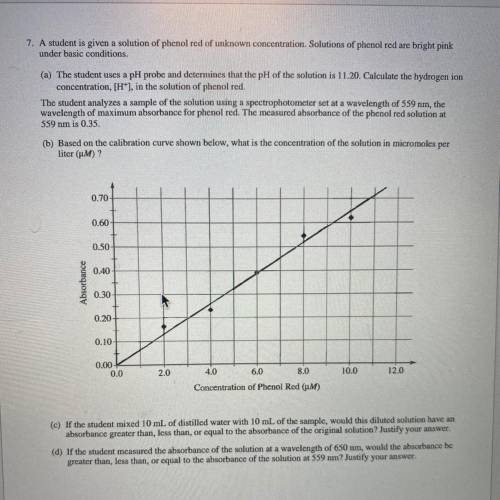
(c) If the student mixed 10 mL of distilled water with 10 mL of the sample, would this diluted solut...
Questions






Mathematics, 14.07.2019 07:30




Social Studies, 14.07.2019 07:30


Mathematics, 14.07.2019 07:30

Mathematics, 14.07.2019 07:30

Social Studies, 14.07.2019 07:30


Mathematics, 14.07.2019 07:30

Mathematics, 14.07.2019 07:30

History, 14.07.2019 07:30