Chemistry, 27.05.2021 22:50 rachellynn02
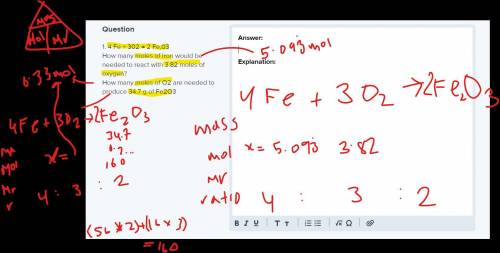
1. 4 Fe + 302 → 2 Fe,03
How many moles of iron would be needed to react with 3.82 moles of oxygen?
How many moles of O2 are needed to produce 34.7 g of Fe2O3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1
You know the right answer?
1. 4 Fe + 302 → 2 Fe,03
How many moles of iron would be needed to react with 3.82 moles of oxygen?<...
Questions