
0.658 g of a compound containing only carbon, hydrogen, and oxygen is burned in excess o2. co2(1.285 g) and h20 (0.658g) are produced. the mol mass of the compound is determined by mass spectrometry to be 90 g/mole determine the empirical and molecular formulas. 20: 0-8453x(12.01s /uu. o 032 029/1b. a mal 1 2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
0.658 g of a compound containing only carbon, hydrogen, and oxygen is burned in excess o2. co2(1.285...
Questions

Business, 22.05.2021 19:40


Mathematics, 22.05.2021 19:40



English, 22.05.2021 19:40




Social Studies, 22.05.2021 19:40


Mathematics, 22.05.2021 19:40


Mathematics, 22.05.2021 19:40






Mathematics, 22.05.2021 19:40

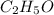
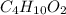
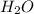 = 0.658 g /18 g/mol = 0.03656 moles
= 0.658 g /18 g/mol = 0.03656 moles
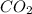 = 1.285 g
/44.01 g/mol = 0.029197 moles
= 1.285 g
/44.01 g/mol = 0.029197 moles


