
BONUS QUESTION
you are stuck with a problem. You need to measure pH of a solution known to be made from a metal hydroxide, but you don't have a meter or any indicators. You do happen to have some lead(II) nitrate that is soluble, and you remember that lead (II) hydroxide is insoluble. You add some to 1 liter of your own unkown solution and a precipitate forms. You add more unttil the precipitate stops forming and then a bit more just in case. After you filter and dry the precipitate, you have 3.81 grams of it. What was the approximate pH of the original solution?
*This is just a bonus question but I'm very confused on it so any help would be appreciated*

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
BONUS QUESTION
you are stuck with a problem. You need to measure pH of a solution known to be made...
Questions

Computers and Technology, 10.11.2020 03:20

Social Studies, 10.11.2020 03:20



Physics, 10.11.2020 03:20



Mathematics, 10.11.2020 03:20

Mathematics, 10.11.2020 03:20


Mathematics, 10.11.2020 03:20


History, 10.11.2020 03:20



English, 10.11.2020 03:20

Arts, 10.11.2020 03:20

Mathematics, 10.11.2020 03:20


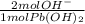 = 0.0316 mol OH⁻
= 0.0316 mol OH⁻

