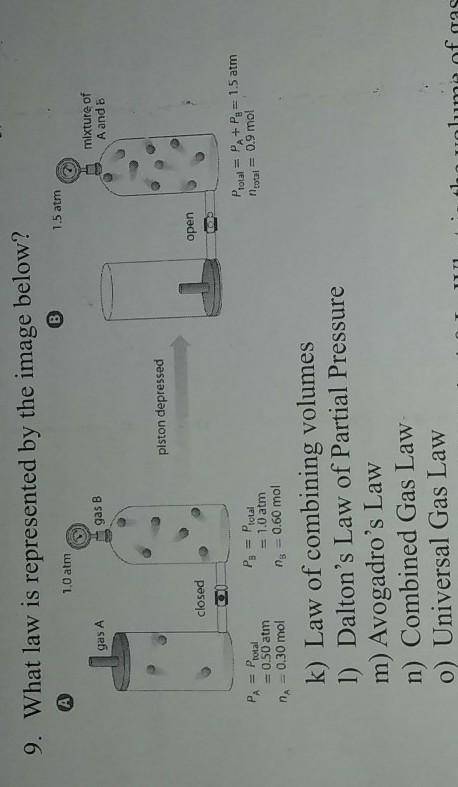
9. What law is represented by the image below?
k) Law of combining volumes
l) Dalton's Law of...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1

Chemistry, 23.06.2019 08:30
Of the following elements, which is the least reactive? a. c b. h c. li d. he
Answers: 1
You know the right answer?
Questions

Arts, 21.01.2020 19:31


Mathematics, 21.01.2020 19:31

Mathematics, 21.01.2020 19:31





Biology, 21.01.2020 19:31


Mathematics, 21.01.2020 19:31


Mathematics, 21.01.2020 19:31

History, 21.01.2020 19:31