
Chemistry, 21.05.2021 22:30 joelpimentel
A spirit burner used 1.00 g methanol to raise the temperature of 100.0 g water in a metal can from 28.00C to 58.0C. Calculate the heat of combustion in kJ/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
A spirit burner used 1.00 g methanol to raise the temperature of 100.0 g water in a metal can from 2...
Questions

Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01



Mathematics, 28.09.2020 14:01

Chemistry, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01


Computers and Technology, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01




Mathematics, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01


English, 28.09.2020 14:01

History, 28.09.2020 14:01

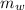 = 100 g
= 100 g



