
Chemistry, 21.05.2021 19:00 logan541972
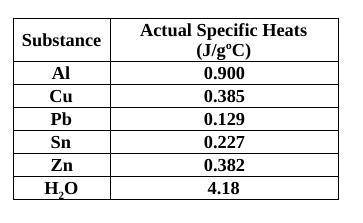
You had a 69.24 g piece of unknown metal and placed it into hot water at 103°C. You then performed the same experiment as done in class (specific heat of a metal lab) using a Styrofoam cup calorimeter (filled with 75.0 mL of water) and you found that the temperature of the water in the cup rose from 23.0˚C to 27.6˚C, what would the metal’s specific heat be? Identify the metal from the table. (The specific heat of water is 4.184 J/g) I need work to be shown

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
You had a 69.24 g piece of unknown metal and placed it into hot water at 103°C. You then performed t...
Questions

Chemistry, 26.09.2019 19:50



Mathematics, 26.09.2019 19:50





History, 26.09.2019 19:50










English, 26.09.2019 19:50



